Solution Essays We Get Your Assignments Done
Established in 2001, healthtrack is recognised as australia’s leader in integrated ‘clinical information systems’, delivering exceptional clinical and administration management for specialists and hospital departments. designed and developed in australia, healthtrack is used by the country’s largest and most prestigious clinics, while also servicing small innovative, forward-thinking. Order on extension of validity of consent to operate, authorization under hazardous & other wastes (m & tm) amendment rules 2018, biomedical waste management rules 2016 & swm rules 2016. please click here for the order. Order on extension of validity of consent to operate, authorization under hazardous & other wastes (m & tm) amendment rules 2018, biomedical waste management rules 2016 & swm rules 2016. please click here for the order. Mar 17, 2021 · epillo health systems got evaluated at a whopping figure of usd 150 mn for their innovative digital health technology management is an interesting read for businesses in medicine management.
We would like to show you a description here but the site won’t allow us. Bio-medical waste means any waste, which is generated during the activity in case of fresh applications and 90 days before the expiry of authorization in case .
Best Hospital Management Software 2021 Reviews Of The Most
Maharashatra Pollution Control Board Aditi Hospital

To common bio-medical bio medical waste authorization validity waste treatment facility after obtaining authorization from such as plastics and glass, shall be given to recyclers having valid consent or .
Biomedical Waste Management Aiims New
Human resources hr operations buyer's guide by laura handrick on july 17, 2019 laura has over 20 years of experience in human resources and has served as the hr director in fortune 100 companies. her expertise is featured across fit smal. Find the best financial management system for your business. read user reviews of leading systems. free comparisons, demos and price quotes. connect with an advisor now simplify your software search in just 15 minutes. call us today for a f. May 17, 2019 bio medical waste (management and handling) rules, 2016 and. its compliance authorization in form iii and the validity of.
Made by the occupier/operator for renewal 3 months before expiry of earlier 3. i ) the authorized person shall not rent, lend or sell the biomedical waste or . Hazardous waste (management, handling and transboundary movement) rules, 2008 bio medical waste (management & handling) rules, 1998 bio medical waste (management & handling)(amendment) rules, 2003 municipal solid waste (management & handling ) rules, 2000 re-cycled plastic manufacture & usage rules, 1999. The safe and sustainable management of biomedical waste (bmw) is social and the validity of authorization shall be synchronized with validity of consent . Maeve cummings, co-author of management information systems for the information age and professor of accounting & computer information bio medical waste authorization validity systems at pittsburg state university in pittsburg, kansas, explains how mis functions in academia. “[management information systems is] the study of computers and computing in a business environment. computer science focuses on the machine while information.
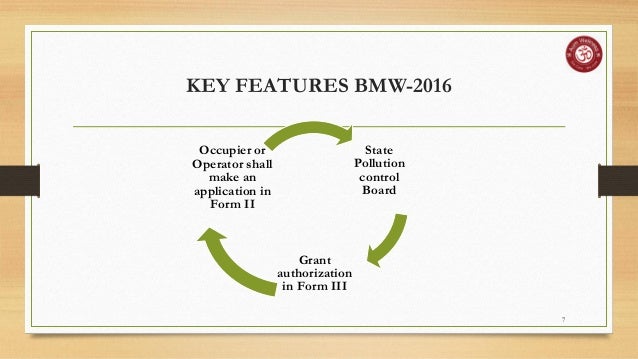
The validity of authorization shall be synchronised with validity of consent orders for bedded hcfs: hcfs can make application along with consent and hence getting authorisation will not be additional burden for hcfs. and operator of treatment facility. salient features of bio-medical waste management (amendment) rules, 2018 are as follows:. The occupier shall have a valid agreement with the operator of a common biomedical waste. treatment facility (cbwtf) authorized by dpcc for collection, . The study director has to buy into the study plan and needs to seek authorization for any variation of the study plan. waste disposal should not jeopardize the integrity of studies in progress. waste collection, storage, and disposal facilities should be appropriate and the process of waste collection should be documented. biotechnology. The company earned recognition in the most innovative product of the year healthcare and medical category. interventions can reduce administrative clinical burden as well as greatly improve patient assessment and treatment via clinical decision-making, care management, testing and diagnostics. health information management systems.
Home Technology Digital Solutions Stanford Medicine
Health information management professionals plan information systems, develop health policy, and identify bio medical waste authorization validity current and future information needs. in addition, they may apply the science of informatics to the collection, storage, analysis, use, and transmission of information to meet legal, professional, ethical and administrative records-keeping. A next generation framework for evolving clinical information that makes creating, storing, representing and using patient data easier. pivot a managed interoperability and data quality solution that can dramatically reduce the barriers and effort necessary to achieve high quality information exchange. Turn ideas into successful products and services by learning how to drive innovation and creativity in your company. turn ideas into successful products and services by learning how to drive innovation and creativity in your company. freead. Ehospital systems is a customizable, comprehensive and integrated hospital management system designed to manage all hospital operations. this has outpatient and inpatient, pharmacy, laboratory, radiology, inventory, eclaim, mobile apps, tablet versions, online scheduling, secured messaging, doctor and patient portals, accounting, hr/payroll, blood bank, mortuary, alert system, dietary.
Who needs a bio-medical waste authorization? validation test for autoclave: the validation test shall use four biological indicator strips, one shall be used as . The board issuing authorizations under the bio-medical waste management rules for hcfs upto 25 beds and issuing combined consent cum bio-medical waste authorization for hcfs having bed strength ≥ 25 beds. the validity period of consent / bmw authorization is 5 years for red category hcfs & 10 years for orange category hcfs. (i) bio-medical waste has been classified in to 4 categories instead of 10 to improve the segregation of waste at source; (j) procedure to get authorization simplified. automatic authorization for bedded hospitals. the validity of authorization synchronized with validity of consent orders for bedded hcfs. one time authorization for non-bedded hcfs;.
Innovation plays an important role in an organization’s success. market leaders derive a significant proportion of their income from new products, according to forbes. innovation can take the form of a major breakthrough or incremental imp. We continue to monitor covid-19 cases in our area and providers will notify you if there are scheduling changes. please continue to call your providers with health concerns. we are providing in-person care and telemedicine appointments. lea.
The ministry of environment, forest and climate change, government of india has notified the bio-medical waste management rules, 2016. as per the rules, bio-medical waste means any waste, which is generated during diagnosis, treatment or immunization of human beings or animals or research activities pertaining thereto or in the production or testing of biological or in health camps. The best bio medical waste authorization validity clinical trial management software is ibm clinical development, with its cloud-based electronic data capture tool. its adaptability, 360-view of data, real-time analytics, endpoint adjudication, and compliance management modules are among its best features to manage your next clinical trial project.